Ten major reprocessing processes including cleaning, disinfection, and sterilization of oral instruments
preprocessing
In order to improve the cleaning quality of dental instruments, it is very important to pretreat the instruments in the clinic. After treatment, the used small instruments should be wiped with dressings or alcohol cotton balls beside the chair to remove blood stains, stains, adhesives, filling materials and other residues on the surface of the dental instruments.
The instruments should be stored moisturized or sealed and sent to the disinfection room as soon as possible to prevent dirt from drying out and affecting the quality of cleaning and disinfection.
Equipment recycling classification
When recycling the equipment, it is necessary to seal it and keep it moisturized, especially the working end. It can be placed in a recycling cleaning rack containing a multi-enzyme solution, and placed in a recycling box with a lid for temporary storage; spray-type enzyme moisturizer can also be used for moisturizing treatment. . Recycling containers should be cleaned, disinfected, and dried after each use.
Pay attention to personal protection when recycling, check whether the gloves are damaged, and take standard precautions. Do not touch the sharp points directly with your hands to avoid needle stick injuries. Small instruments such as burrs and mobile phones should be handled separately.
Manual cleaning steps
Rinse: Rinse the instrument under running water to initially remove contaminants.
Brushing and scrubbing: The rinsed instruments need to be soaked in enzymatic cleaning fluid. During soaking, the liquid level must cover the instruments to keep them fully moist. Then brush and scrub.
Final rinse: After brushing and scrubbing, rinse with purified water.
Ultrasonic cleaning
- Ultrasonic cleaning machine can improve the cleaning effect and efficiency.
It is suitable for instruments that are difficult to clean such as blood-stained dental forceps, burs and other instruments with complex structures. The ultrasonic cleaning time should be 3min-10min. The cleaning time can be appropriately determined according to the contamination of the instrument and the power of the ultrasonic machine.
Note: Ultrasonic cleaning is only an auxiliary cleaning to manual cleaning. It cannot shake the mobile phone to clean it, as it can easily damage the bearings, especially expensive mobile phones (such as planting mobile phones).
- Mobile phone equipment is recommended to be cleaned with a medical cleaning machine.
Dental handpieces are precision manufactured and have complex structures. They are in close contact with patient saliva, blood, etc. during diagnosis and treatment, so special equipment is used for cleaning.
Cleaning process:
Rinse under running water to remove any obvious dirt on the surface of the dental handpiece. Put the dental handpiece into the mechanical cleaning equipment, fix the dental handpiece, select the correct cleaning program, and start the equipment to start cleaning. Its cleaning water flow and air flow conform to the internal structure of the dental handpiece.
Mobile phone oiling and maintenance
It is recommended to configure a special automatic oiling machine for dental handpieces
Instructions for use: Select the oil filling time and machine position. Start the cleaning and oiling process. After the oil filling is completed, start the drying process. Time: 35 seconds each. Improve work efficiency. Enclosed devices reduce contamination.
Oral mobile phone oiling and maintenance:
Oil injection is lubrication. Its function is to clean debris in the clearance of bearings or turbine components and lubricate bearings and transmission components. Frequent oiling can greatly extend the service life of your mobile phone. Oil filling should be done after cleaning and before sterilization to protect the phone from being sterile and avoid contamination before use. The inner cavity of the mobile phone should be kept dry before filling with oil.
Instrument inspection
Dry oral instruments should be inspected visually or using a lighted magnifying glass.
Detail standards: The surface of the instrument, the spiral structure, and the joints should be free of stains, water stains and other residual substances and rust spots. Instruments that fail to be cleaned should be reprocessed. Damaged or deformed instruments should be reported and replaced in a timely manner.
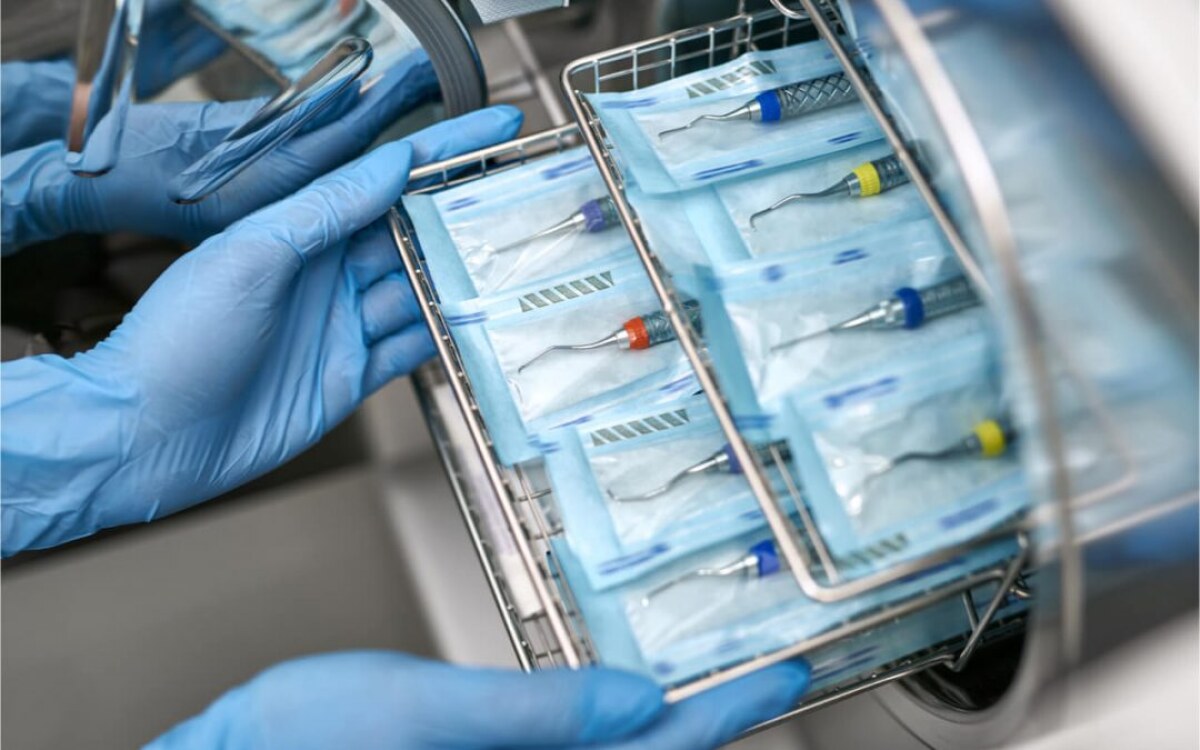
Device packaging sealing
Packaging requirements for oral instrument sterilization bags:
Packaging materials should be selected based on device characteristics and frequency of use.
Low- and medium-risk oral instruments may not be packaged, and may be stored directly in spare clean containers after disinfection or sterilization. Small dental instruments should be packed in dental instrument boxes.
Paper-plastic packaging sealing requirements are as follows:
There should be a sterilization chemical indicator on the outside of the package, and it should be marked with the name of the item, the packager, the sterilizer number, the sterilization batch, the sterilization date and the expiration date. If there is only one sterilizer, the sterilizer number does not need to be marked. . Paper-plastic packaging should be completely sealed, the sealing width should be ≥6mm, and the distance between the instruments in the package and the seal of the packaging bag should be ≥2.5cm.
It is recommended to use a fully automatic sealing machine, which can effectively avoid uneven manual pressure and cause damage to paper and plastic bags. It can also print sealing time, expiration date, operator, pot number, equipment name and other information.
Cotton fabric sterilization packaging requirements:
It cannot be stiff or damaged. Seal with sterilization tape. A chemical indicator card or “crawl” card must be placed in the bag. The wrapping cloth needs to be cleaned once every use, and the quality of the wrapping cloth should be checked every time it is used. It is suitable for all dental diagnosis and treatment instruments, and is more suitable for larger and larger surgical instruments.
Disinfection and sterilization
Loading principles:
A reasonable weight of loaded items should be selected based on the size of the sterilizer cavity. The weight of loaded items should be subject to the manufacturer’s instructions. The loaded items should ensure smooth circulation of steam in the sterilization chamber. This keeps the temperature uniform throughout the interior.
It should be conducive to the discharge of air from the sterilization chamber, so as to ensure that the steam pressure and temperature in the container are consistent. Do not block temperature, pressure and other sensors to avoid affecting the normal operation of the sterilizer.
Loading method:
Sterilization items should not be stacked closely to prevent blocking the sensor measurement and control ports. After clogging, the sterilizer will not be able to correctly reflect the temperature in the sterilization chamber. Continuous temperature rise may cause abnormal pressure and temperature in the sterilization chamber, and even lead to sterilization failure.
In severe cases, it may cause sterilizer failure. For lidded containers, the lid should be opened to allow air and steam to exchange from the gap. If the lid is too tight, the container may easily burst during sterilization.
How to place paper-plastic packaging bags:
Using a bracket and a pallet, the paper-plastic packaging bags are laid flat on the pallet, with the paper on top and the plastic on the bottom.
Chemical instruction cards and related records
The results presented by each sterilization indicator should be systematically recorded and traceable. The monitoring data and records should be kept for ≥3 years to facilitate reference in case of problems.